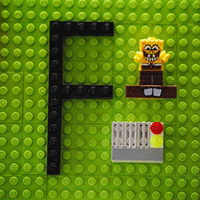When a fluorine atom gains an electron, it becomes a fluoride ion:
F + electron –> F–
Teeth are often treated with fluoride. This is because when fluoride substitutes for hydroxide in hydroxyapatite (the mineral in teeth), a stronger mineral results:
Ca10(PO4)6(OH)2 + 2 F– –> Ca10(PO4)6(F)2 + 2 OH–